Introduction: Youth with sickle cell disease (SCD) and their families are susceptible to stress and depression associated with chronic illness and social factors disproportionately affecting under-resourced U.S. communities. The COVID-19 pandemic has adversely impacted psychosocial and economic well-being, especially in some of these same communities. Our concurrent HABIT multi-site randomized trial aims to improve hydroxyurea adherence in youth with SCD ages 10-18 years through an intervention led by community health workers(NCT03462511). Subjects enrolled as youth-primary caretaker dyads; adults were mostly parents. We hypothesized that some HABIT subjects had depressive symptoms at baseline, and many had additional stressors during the pandemic.
Methods: Two self-reported assessment tools were used, with options of English or Spanish: 1) PROMIS® pediatric (8a, v1.0) or adult (4a, v1.0) depression measures, completed at HABIT enrollment, nearly all between May 2018 - March 2020 ("baseline"); 2) A pandemic-related open-access survey originated by Johns Hopkins University on established core adult mental health assessed risks and behaviors.[1] Questions were closely adapted for use by youth. The pandemic survey assessed recent mental health symptoms and substance or domestic abuse. Two validated food insecurity screening questions were added.[2] Of 92 HABIT subjects, 84 were offered survey participation between May - July, 2020. Participants completed both assessment tools via electronic linkage to REDCap data capture. Analyses used chi square or Fisher exact test.
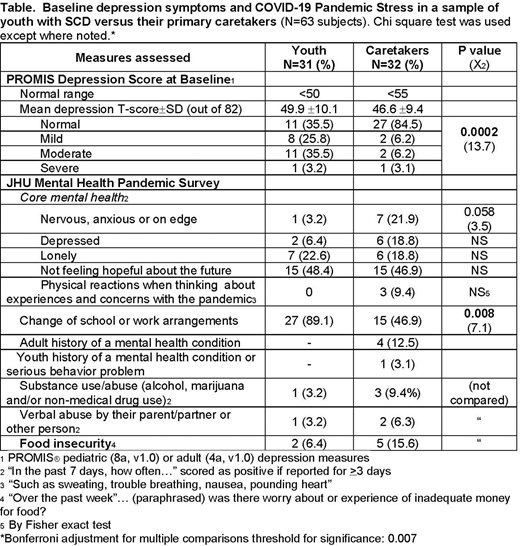
Results: In all, 75% (63 of 84) responded to the pandemic survey; 31 were youth-parent dyads and one unpaired parent. Baseline demographics were: Youth (N=31): mean age 12.9±1.9 years, 48.4% female, 80.6% grade 6-12, 45.3% hospitalized within the prior 12 months; Caretakers (N=32): mean age 44.0±9.6 years, 87.5% Black, 18.8% Latinx, 37.50% married or living with a partner, 59.4% with at least some college education. At baseline, youth mean PROMIS® Depression T-Score was 49.9±10.1 (normal <50), with 64.5% reporting mild, moderate or severe depressive symptoms, compared to Caretaker' mean score 46.6±9.4 (normal <55) with 15.5% symptomatic (p=0.0002) (Table). In contrast, the pandemic survey revealed that 3 (9.7%) youth and 8 (25.0%) caretakers had recently felt depressed and/or anxious (NS). Loneliness (1 in 5) and especially not feeling hope for the future (1 in 2) were common in both groups. More youth than caretakers (89.1% vs.46.9%) had changes made to their school or work arrangements (p=0.008). Four (12.5%) caretakers and 1 (3.2%) youth had histories of mental illness. Substance use/abuse or verbal abuse were reported in <10% of each group. Food insecurity was reported in 6 (18.8%) families. "Red flag" replies to the pandemic survey necessitated referral of 6 dyads (18.8%) to their SCD social workers for support.
Conclusions: In this sample of subjects from the HABIT Trial, at baseline a higher proportion of youth had depressive symptoms compare to their primary caretakers. During the initial pandemic peak in the Northeast, disrupted work arrangements and especially school cancellation were widespread. Fewer youth but similar proportions of caretakers reported feeling depressed and/or anxious. Both groups commonly reported loneliness or not feeling hopeful for the future. History of mental health conditions, current substance use/abuse or verbal abuse were uncommon. Concordant with concerns for under-resourced communities, a sizeable minority of families reported food insecurity. Under the limitations of using 2 different assessment tools, in this modest sample the majority of youth with SCD but not caretakers were mildly-moderately depressed at baseline and that, during the pandemic, the 2 groups reported similar proportions of mental health symptoms. These findings suggest that screening for mental health symptoms, social disruption and food insecurity may be warranted in this high-risk group overall and during the pandemic.
References:
1) COVID-19 and mental health measurement working group, Johns Hopkins Bloomberg School of Public Health, March 18, 2020
2) Barnidge E., et al., Screening for Food Insecurity in Pediatric Clinical Settings. J. Community Health 42(1):51-57, 2017
The HABIT Trial is supported by 5R01NR017206-04 (Green, Smaldone). The authors have no conflicts to disclose.
Smith-Whitley:Novartis: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Prime: Other: Education material; Celgene: Membership on an entity's Board of Directors or advisory committees. Aygun:bluebird bio: Membership on an entity's Board of Directors or advisory committees, Research Funding; National Institute of Nursing Research: Research Funding; Patient-Centered Outsomes Research Institute: Research Funding; National Heart, Lung, and Blood Institute: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.